Che 31 Introduction To Chemical che 31. INTRODUCTION TO CHEMICAL ENGINEERING (Credit: 3 Units) The course is intended to provide students a clear overview of the field of chemical engineering and introduce them to the elementary principles involved in the analysis of chemical processes with emphasis on material and energy balance calculations. CBE:5120 Data Science in Chemical and Engineering Systems GETTING STARTED ASSIGNMENTS Course Description: Theory and application of numerical methods and data-driven algorithms towards understanding chemical processes; Scientific computing in the Python programming language; Numerical solutions to differential equations; Nonlinear and constrained optimization; data preprocessing. Solutions Manuals are available for thousands of the most popular college and high school textbooks in subjects such as Math, Science (Physics, Chemistry, Biology), Engineering (Mechanical, Electrical, Civil), Business and more. Understanding Introduction To Chemical Engineering 5th Edition homework has never been easier than with Chegg Study.
ICEP
A printable version of Introduction to Chemical Engineering Processes is available. (edit it) |
A PDF version of Introduction to Chemical Engineering Processes is available. 1.59 Mb, 5-08-07,136 pages (info) |
This book is intended for advanced readers. |
Chapter 0: Introduction[edit]
- Purpose of the Book
- Formatting of the Book
- Invitation to Contribute
Chapter 1: Prerequisites[edit]
Units
- Base unit types
- Consistency and Systems of units
- How to convert between units
- Dimensional analysis as a check on equations
Significant figures
- Why do they matter? The parable of an expensive cement block.
- How to compute them when we're adding
- How to compute them when we're multiplying
- When do you round 5 down? And why not just round it up like the elementary school teachers taught you?
General chemistry review
- Stoichiometry and Moles vs. Grams
- Ideal Gas Law
- Enthalpies and Entropies of Formation
Chapter 1 Practice Problems
Chapter 2: Elementary mass balances[edit]
What is a mass balance?
- Black box approach
- Conservation of mass
- General conservation equation
- How that conservation equation will apply to this class
- Some notation
- The final result
Converting Information into Mass Flows
- Introduction: Easily-measurable vs. derived variables
- Volumetric flow and Density
- Velocity and Cross-sectional Area
- Mass flows vs. Molar flows
- More complex example, 1-component streams and multiple operations
- Drawing flowcharts
- Some basic conversions and strategies
- Overall balances vs. balances on single operations
Chapter 3: Mass balances on multicomponent systems[edit]
The most important point:
- Mass of each species is conserved (when there's no reaction)
- The use of concentrations and total flows
- Bulk mixture properties


- General methods and tips
- Conversions between Units.
- Example Problem with Solution
- Things in reality are often considerably more complex.
- Degree of Freedom Analysis.
- Example of a more complex problem
Chapter 4: Mass balances with recycle[edit]
- Conserve resources
- Increase yield
- Save space
- Most importantly, save money
- Use of combination and splitting points in balances
- Words of caution with regards to degree of freedom analysis
- An example of the effect of recycle on a separation process.
- Displays another application of recycle.
Chapter 5: Mass/mole balances in reacting systems[edit]
- Review of reaction stoichiometry
- Lack of a 'law of conservation of moles'
- Molar extents of reaction
- Steady-State Molecular mole balance equation
- Degrees of Freedom
- Independent and dependent chemical reactions
- Inerts versus Reactive Species
- Equilibrium constants (introduction/review from general chem)
- Extent of Reaction is still Extent of Reaction
- Example Problem without equilibrium
- Example Problem with equilibrium
- Example of how a separation process can be used to improve efficiency.
- Conservation of moles of atoms
- The general idea
- Some specific examples
- Advantages and shortcomings of the atom balance method
Chapter 6: Multiple-phase systems, introduction to phase equilibrium[edit]
- Ideal Gas assumption, not law
- Concept of Equations of State
- Alternative Equations to the Ideal Gas Equation
Filesche 31. Introduction To Chemical Engineering
- Activity Coefficients
- Solubility Coefficients
- Gibbs energy
- Fugacity and fugacity coefficients
- What is different for a mixture?
- Partial properties
- Properties of ideal mixtures (liquid and gas)
- Equality of partial fugacities in all phases at equilibrium
- Ideal gases and ideal solutions (to do: move this to the 'mixtures' section)
- Vapor pressure; Antoinne equation
- Raoult's Law (VLE)
- VLE for non-ideal systems
- VLE charts
- Immiscible fluids
- Miscible fluids: Separation constant, K
- Use of acid/base reactions in separation
- LLE charts
- Critical Constants
- Generalized Forms of the Equations of State
- Generalized Compressibility Charts
- Kay's Rule
- Bubble points and dew points
- Solving for equilibrium concentrations, temperature, and pressure
- Use of equilibrium equations in solving mass balances
Chapter 7: Energy balances on non-reacting systems[edit]
- Idea behind energy balance: conservation of energy
- Types of energy that might be important
- Most common types of energy
- Energy change due to flows
- Energy change due to temperature changes
- Energy change due to PE and KE differences
- Heat and work
- Actual steady state Energy balance equation (for open and closed systems)
- Heat Capacity of an Ideal Gas
- Reference Values
- Methods for calculating heat transfer
- Understanding 'accumulation'
- Unsteady-state mass balance (discretized)
- Discretized unsteady-state mass balance
- A glimpse of things to come
( A note: there are many, many, many different forms of the energy balance out there for specific situations, this one is somewhat general but does not get into internals of the system at all and thus cant tell us about local temperature profiles and stuff like that. Also, this is most useful form for heat-dominated processes; for mostly mechanical processes a different form is more useful)
- Open and Closed-system problems (examples)
Chapter 8: Combining energy and mass balances in non-reacting systems[edit]
- The basic idea
- Most common unknowns
- What is in common between the balances?

- Several example problems illustrating how to combine these two concepts.
- Without phase change
- With phase change
Chapter 9: Introduction to energy balances on reacting systems[edit]
- What's different for a reacting system?
Filesche 31. Introduction To Chemical Engineering Processes
- Incorporating heat of reaction into the energy balance
- Putting it all together: steady-state mass/energy balance problem with reaction(s)
Appendix 1: Useful Mathematical Methods[edit]
- Linear Regression
- Transformation of functions into linear form (linearization)
- Interpolation and Extrapolation
- Fixed Point and Weighted Fixed-Point Iteration
- Bisection Method
- Regula Falsi
- Tangent (Newton) Method
- Explanation of Systems and Solvability
- General strategies
- Specific instances: linear equations, quadratic equations
- Example Solutions from This Text
- How to choose a scale
- Linearization's use in graphing
- Alternate axes: Log-log and semi-log plots
- Common plot types:
- Parity plots
- Residual plots
Appendix 2: Problem Solving using Computers[edit]
Filesche 31. Introduction To Chemical Engineering Degree
- Data entry
- Manipulation and Graphing of Data
- Regression Analysis
- Goal seek
- Solving systems of equations with symbolic math toolbox
- Linear and Polynomial Regression
- Plotting functions and data points
Appendix 3: Miscellaneous Useful Information[edit]
- Direct measurement methods for flow rates (bucket and timer, flow meters, etc.)
- Measurement methods for pressure or pressure drop (manometer, barometer, etc.)
- Measurement of velocity (orifice meters, venturi meters, etc.)
- Measurement of concentration (GC, titrations, etc.)
- Measurement of temperature (types of thermocouples and thermometers)
- Standard vs. Actual Volume
- Types of Moles Other than gram-mole
- Gauge Pressure vs. Absolute Pressure
- Pounds-Force vs. Pounds-Mass
- Equipment Description Summaries
- Links to Further Information

Appendix 4: Notational Guide[edit]
- Notation used in this book
- Warning about non-uniformity of notation across sources
Appendix 5: Further Reading[edit]
Chapra, S. and Canale, R. 2002. Numerical Methods for Engineers, 4th ed. New York: McGraw-Hill.
Felder, R.M. and Rousseau, R.W. 2000. Elementary Principles of Chemical Processes, 3rd ed. New York: John Wiley & Sons.
Masterton, W. and Hurley, C. 2001. Chemistry Principles and Reactions, 4th ed. New York: Harcourt.
Perry, R.H. and Green, D. 1984. Perry's Chemical Engineers Handbook, 6th ed. New York: McGraw-Hill.
Windholz et al. 1976. The Merck Index, 9th ed. New Jersey: Merck.
General Chemistry: For a more in-depth analysis of general chemistry
Matlab: For more information on how to use MATLAB to solve problems.
Numerical Methods: For more details on the rootfinding module and other fun math (warning: it's written at a fairly advanced level)
Himmelblau, D. M. and Riggs, J. B. 2004. 'Basic Principles and Calculations in Chemical Engineering', 7th ed. New York: Prentice Hall
Appendix 6: External Links[edit]
Data Tables
Filesche 31. Introduction To Chemical Engineering Lecture
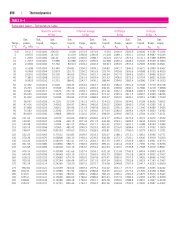
Chemical Sciences Data Tables: Has a fair amount of useful data, including a fairly comprehensive List of Standard Entropies, and Gibbs Energies at 25oC (also a list for ions), a chart with molar masses of the elements, acid equilibrium constants, solubility products, and electric potentials. Definitely one to check out.
NIST properties: You can look up properties of many common substances, including water, many light hydrocarbons, and many gases. Data available can include density, enthalpy, entropy, Pitzer accentric factor, surface tension, Joule-Thompson coefficients, and several other variables depending on the substance and conditions selected. To see the data in tabular form, once you enter the temperature and pressure ranges you want, click 'view table' and then select the property you want from the pull-down menu. It'll tell you acceptable ranges.
Generalized compressibility chart: This is very useful in the section on gases and liquids, and you should be able to find a copy of this chart in any thermodynamics book or in Perry's handbook. I've linked here so you have some clue what I'm talking about when I write about it.
SIRCh: Physical Property Searches (Selected Internet Resources)
Filesche 31. Introduction To Chemical Engineering Flowchart
